What Exactly Does It Mean to Have a Hydrophobic, Oleophobic, or Hydrophilic Surface?
The process of changing the surface of a material by introducing new physical, chemical, or biological features that are distinct from those already present on the surface of the material is referred to as surface modification.
Hydrophobic Effect

A hydrophobic person is afraid of water (hydro) (phobic). In chemistry, hydrophobicity refers to the quality of a molecule, also known as a hydrophobe, that causes it to be repulsed by a collection of water. This is entirely due to the lack of attraction and does not include any repelling force. The use of alkylsilanes on a wide variety of mineral substrates can produce hydrophobic effects, including but not limited to the following:
- Porous surfaces like concrete and natural stone are utilised to generate a water-repellent effect.
- Mineral fillers and pigments enhance organic polymers’ dispersibility and compatibility with one another.
Oleophobic Effect
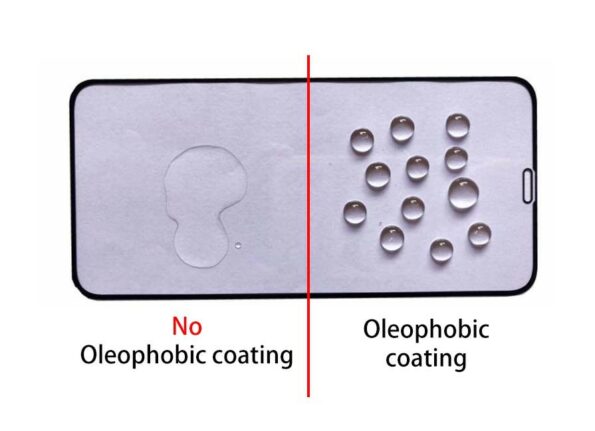
The term oleophobic, the fear of oil, or lipophobia, is meant to be conveyed, which can also be used interchangeably. It is a feature of chemical compounds that translates to “fear of fat,” and its name means “fat rejection.” Compounds are said to be oleophobic or lipophobic when they cannot dissolve in lipids or other non-polar solvents. They do not take in lipids like other organisms do. Fluoroalkylsilanes, when applied to a wide variety of substrates, can confer oleophobic properties on the surface of the treated material.
- Glass and ceramic are two examples of smooth surface materials that can be used to generate long-lasting anti-stick and easy-to-clean qualities.
- Concrete and natural stone are examples of porous surfaces that can be used to produce anti-graffiti qualities and are also easy to clean.
- Cellulose-based materials, such as wood, achieve durable water and oil repellence.
Hydrophilic Effect
The word “hydrophilic” literally means “loving water” (hydro) (phillic). Hydrophilic compounds, such as salts, have the potential to give the appearance of drawing moisture out of the surrounding air. In the same way as salt does, sugar, which is also hydrophilic, can be utilized to extract water from foods. Using silanes with hydrophilic functionalities, such as amino, polyethene oxide, or epoxy, is one way to produce hydrophilic effects on mineral materials. These effects can be produced through the use of silanes. These characteristics allow for increased filler loadings while simultaneously improving dispersibility in polar solvents, water, and polar polymers.
Organophilic Effect

In its simplest form, the term “organophilic” refers to a chemical or molecular orientation that favours the attraction of hydrocarbons or substances that are miscible in hydrocarbons. Weak dipole moments are characteristic of organophilic materials. In water, they are essentially insoluble. Several organofunctional silanes can be used to give inorganic materials an organophilic effect.
- These qualities are useful in a wide variety of contexts, including:
- compatibility of the organic polymer with the various types of filler and reinforcing
- Improve processing
- Higher filler loadings
- An improved wetting of the inorganic material by the polymer
How Do Liquids Function, You Ask?
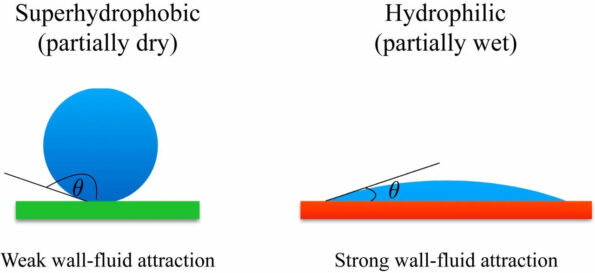
Liquids, in contrast to solids, tend to adhere to various surfaces, including themselves. This adhesion behaviour is characteristic of liquids. How a surface engages with liquid, more especially water droplets, is used to determine whether or not it is hydrophobic. Which means it is afraid of water, or hydrophilic, which means it is drawn to water.
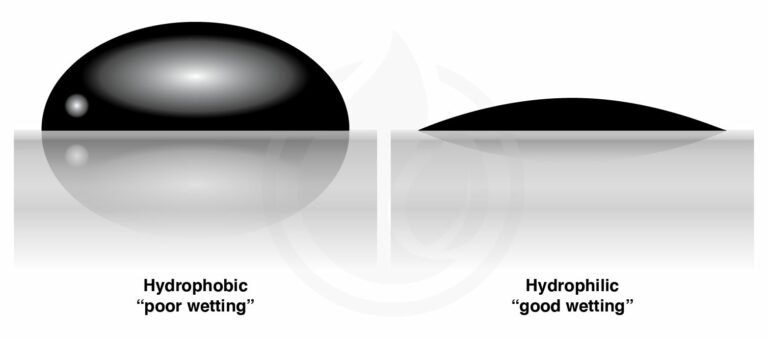
- A water droplet that thoroughly wets the surface has a contact angle of 0 degrees, but a droplet that has no interaction with the surface would bead up into a perfect sphere with a contact angle of 180 degrees.
The Significance of the Contact Angle
The water droplet will have a contact angle of less than 90 degrees if the surface it rests on is hydrophilic, and the water droplet will stick to the surface more than it sticks to itself.
If the water droplet has a greater tendency to adhere to itself than it does to the surface, the surface in question is hydrophobic. Water droplets will bead up to a contact angle of more than ninety degrees.
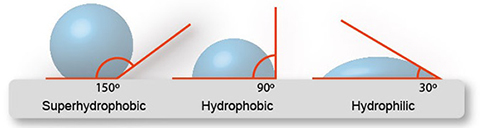
When the contact angle of water drops exceeds 150 degrees, a surface is said to be superhydrophobic. There are many naturally occurring examples of surfaces that are very resistant to water. These include the surfaces of plants as well as those of insects.
One of the most well-known examples of a superhydrophobic surface seen in nature is the surface of a lotus petal. Researchers initially became interested in studying superhydrophobic events due to the excellent water repulsion displayed by lotus leaves.

One surface is said to have the property of being superhydrophobic if it has contact angles of more than 150 degrees in both the advancing and retreating directions.
The roll-off angle refers to the angle at which a water drop rolls off a slanted flat surface. The roll-off angles of superhydrophobic surfaces are typically fewer than 5 degrees. Although very high-quality surfaces can display roll-off angles of less than 1 degree.
Why is it so important that something be hydrophobic?
Because it is hydrophobic, something that repels water will cause drops of water to form. Because of their hydrophobicity, surfaces have a higher likelihood of self-cleaning when water is put to them. This is crucial.
In addition, it has been demonstrated that a hydrophobic surface can greatly improve visibility and clarity while maintaining a relatively clean appearance after being subjected to several weathering tests.
Drag reduction experiments have demonstrated not only a reduction in the cost of transporting products by ship but also an improvement in the efficiency of watercraft of all types.
Hydrophobic surfaces can also significantly minimise or eliminate many of the impacts of ice storms and aircraft icing. These benefits are especially useful in colder climates. Icicles are prevented from developing on coated surfaces because ice formation is drastically decreased on these surfaces.







